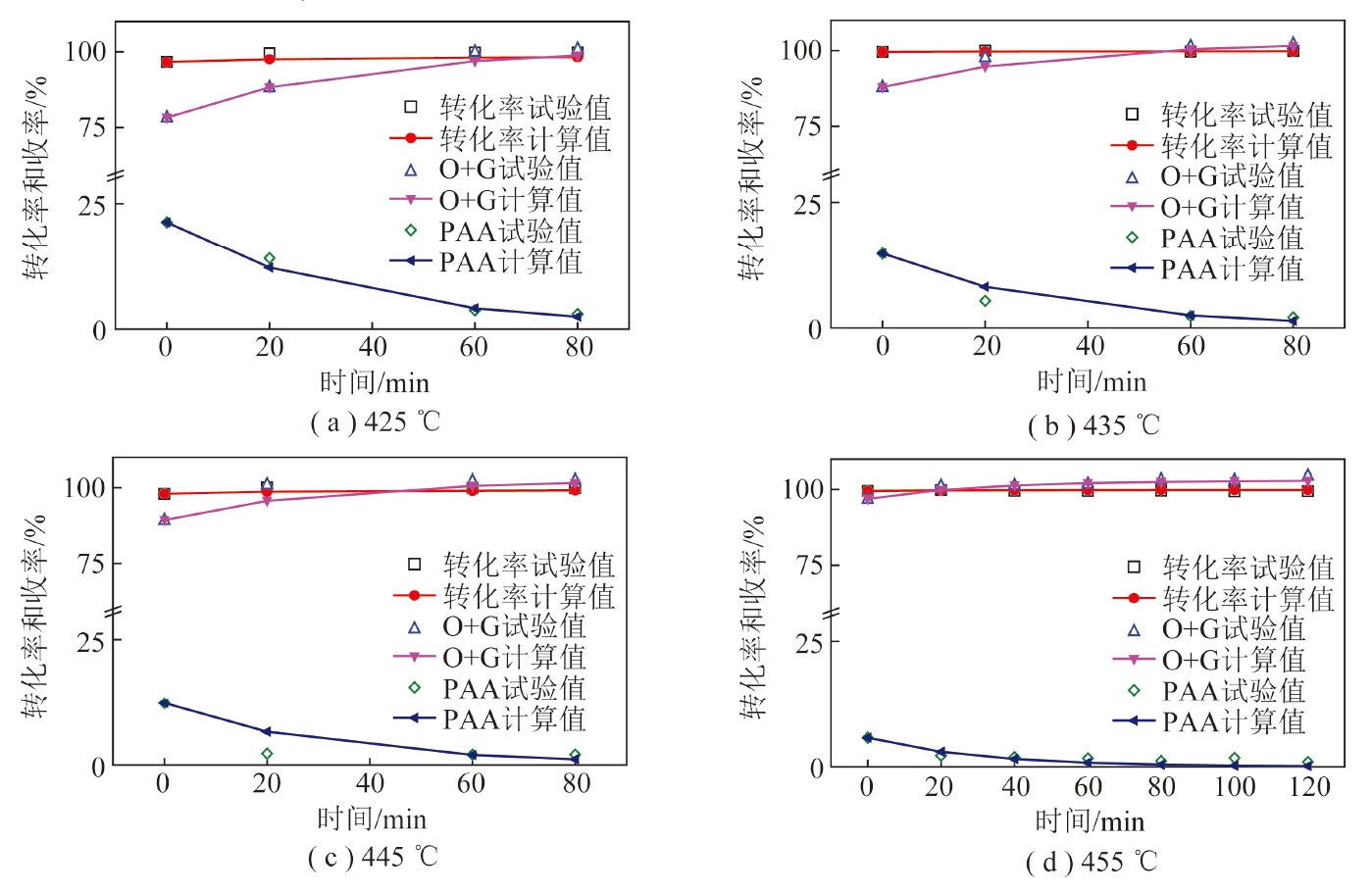
In order to study the direct liquefaction characteristics and product distribution of Naomaohu coal in Xinjiang,the effects of reaction temperature and reaction time on the yield of liquefied products were investigated in the 0.5 L batch autoclave with tetrahydronaphthalene as solvent,nano-iron oxide as catalyst and S as a promoter. The results show that Naomaohu coal is easy to be liquefied. When the reaction temperature just rises to 425 ℃,the conversion is up to 96.6%,and the oil yield is 56.68%. With the increase of reaction temperature and reaction time,coal conversion,hydrogen consumption,gas yield,and oil yield gradually increase,while the yield of asphaltic substances decreases and the water yield remains basically unchanged. Then,as the reaction temperature increases further and the reaction time continues to extend,the light oil will be further cracked,leading to further increase in gas yield and decrease in oil yield. When the reaction temperature is 455 ℃ and the reaction time is 80 min,the conversion rate of coal reaches 99.6%,the yields of oil,asphaltic substances,and gas are 73.42%,1.64%,and 16.61%,respectively,and the hydrogen consumption is 4.85%. According to the law of liquefaction reaction,five lumped reaction kinetic models were established. The Kinetic model parameters were obtained by using the optimization algorithm. The relative errors of the simulated and experimental values of coal conversion,asphalt substances,and oil and gas yield are 0.5%,1%,and 8%,respectively.
Study on the direct liquefaction behaviors of Xinjiang Naomaohu coal
 2021 No. 04
2021 No. 04
 685
685 477
477

Authors:
- SHAN Xiangen
- SHU Geping
- CAO Xueping
- WANG Hongxue
- GAO Shansong

Unit:
- National Engineering Laboratory for Direct Coal Liquefaction,China Shenhua Coal to Liquid and Chemical Company
- Shanghai Research Institute

Abstract:

Keywords:
- direct coal liquefaction
- bituminous coal
- reaction properties
- kinetic model

Citation format:
单贤根(1982—),男,江苏盐城人,高级工程师,博士,研究方向为煤直接液化。E-mail:xiangen.shan@chnerengy.com.cn

Chart:

Articles:
--

Citation format:
--

-
Executive director
China Coal Science and Industry Group Co., Ltd
-
Sponsored by
Coal Science Research Institute Co., Ltd
Coal Industry Clean Coal Engineering
Technology Research Center -
Editor in Chief
XIE Qiang
-
Vice Editor-in-Chief
YU Chang
SHI Yixiang
ZHAO Yongchun
DUAN Linbo
CAO Jingpei
ZENG Jie -
Publication Frequencies
Monthly
-
ISSN
1006-6772
-
CN
11-3676/TD
Covered by
- CSTPCD
- RCCSE(A+)
- AJ
- EBSCO host
- Ulrichsweb
- JST
- Scopus
Contact us
New Media
-
 Meichuanmei
Meichuanmei -
 Clean Coal Technology
Clean Coal Technology -
 Online Journals
Online Journals






 Submission system
Submission system Copyright agreement
Copyright agreement Instructions for authors
Instructions for authors