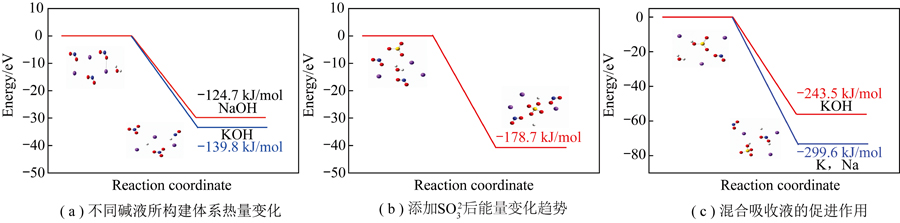
The flue gas absorption test was simulated using KMnO4 and NaClO2 as oxidants, and the effects of single alkaline absorptionsolutions and Na-K synergistic absorption solutions on the removal of NOx from flue gas were investigated. Trace amounts of SO2-3 ions wereintroduced during the process to further optimize the absorption efficiency. The results indicate that the performance of KOH alkaline solution is significantly higher than that of NaOH. The average absorption rate of KOH within 30 minutes of absorption is as high as 99.4%,while NaOH only achieves an average absorption rate of 86.9%. This difference can be attributed to the larger ionic radius of K+ ions,which facilitates the dissociation of OH- ions. When using 0.1 mol/ L KMnO4 as the oxidant and a two-component absorption solution (n(Na) ∶ n(K)= 1 ∶ 2), the efficiency is higher and more stable, reaching 86% even after 30 minutes. It is due to the charge transfereffect of bimetallic ions, which results in the rapid rupture of the gas film when gas-phase reactants transfer to the liquid phase, reducingthe diffusion time between the gas and liquid phases. This phenomenon has been thoroughly confirmed through density functional theory(DFT) simulation calculations. The average absorption rate is increased by 3% by introducing trace amounts of SO2-3 ions into the absorption solution, demonstrating that SO2-3 ions further promote the absorption and conversion of nitrate species.
Influence mechanism of Na-K synergism on the evolution of NOxin wet NO reduction
 2023 No. 10
2023 No. 10
 407
407 320
320

Authors:
- SHAO Ranlei
- HAN Shiwang
- XUAN Chengbo
- WANG Luyuan
- ZHANG Xingyu
- CHENG Xingxing
- WANG Zhiqiang

Abstract:

Keywords:
- Na-K synergy
- wet denitrification
- NO peroxidation
- ion chromatography

Citation format:
邵然磊(2000—),男,山东烟台人,硕士研究生。E-mail:19862129133@163.com

Chart:

Articles:
--

Citation format:
SHAO Ranlei,HAN Shiwang,XUAN Chengbo,et al.Influence mechanism of Na-K synergism on the evolution of NOx inwet NO reduction[J].Clean Coal Technology,2023,29(10):166-175.

-
Executive director
China Coal Science and Industry Group Co., Ltd
-
Sponsored by
Coal Science Research Institute Co., Ltd
Coal Industry Clean Coal Engineering
Technology Research Center -
Editor in Chief
XIE Qiang
-
Vice Editor-in-Chief
YU Chang
SHI Yixiang
ZHAO Yongchun
DUAN Linbo
CAO Jingpei
ZENG Jie -
Publication Frequencies
Monthly
-
ISSN
1006-6772
-
CN
11-3676/TD
Covered by
- CSTPCD
- RCCSE(A+)
- AJ
- EBSCO host
- Ulrichsweb
- JST
- Scopus
Contact us
New Media
-
 Meichuanmei
Meichuanmei -
 Clean Coal Technology
Clean Coal Technology -
 Online Journals
Online Journals













 Submission system
Submission system Copyright agreement
Copyright agreement Instructions for authors
Instructions for authors